Physics teaches us that water, when evaporating in an adiabatic system, removes sensible heat from such system. The temperature thus decreases. In order for the water to evaporate, energy is required, specifically 2501 kilojoules for each kilogram of evaporated water. In the absence of an external energy source, the water absorbs the required energy from the environment, in this case from the surrounding air. As a consequence, the air is cooled. In simple terms, it can be stated that evaporation of water converts the sensible heat of the air (temperature) into latent heat (humidity). As a result, the system is cooled and humidified at the same time. This type of cooling is referred to in literature as evaporative cooling or adiabatic cooling.
Evaporative Cooling on the Psychrometric Chart
Psychrometric charts show the air humidity ratio on the Y axis, and the dry bulb temperature, i.e. the temperature measured by an ordinary thermometer, on the X axis. The curved lines connect points with the same relative humidity (e.g. 40%, 60%, 80%rh).
 Figure 1. Psychrometric chart
Figure 1. Psychrometric chart
At a certain temperature, the air contains a certain quantity of water vapour. When the quantity of vapour is at the maximum (100%rh), saturation point has been reached and the vapour starts to condense. The saturation points are joined by the saturation curve. The higher the temperature, the more water vapour the air can contain. The following example explains the effect of evaporative cooling. The dry bulb temperature is 40 °C, and the relative humidity is 15% (the air is not saturated). By allowing water to evaporate spontaneously, the temperature of the humid air will fall until reaching the saturation curve. The heat in the air is absorbed by the water as it evaporates: sensible heat is converted into latent heat. The effect in the example is that the temperature falls by 20 °C. This psychrometric transformation is represented by a segment that joins two points with the same enthalpy, i.e. the same amount of energy, as sensible heat is transformed into latent heat yet the sum of these remains constant.
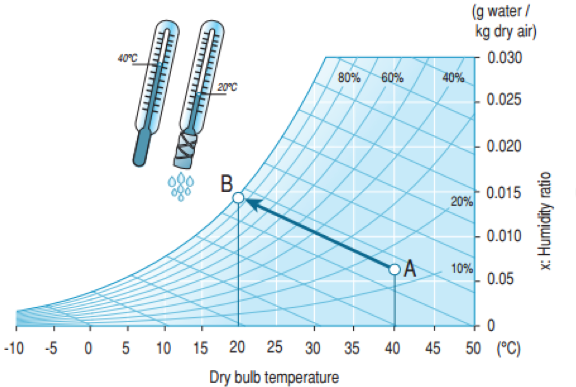 Figure 2. Adiabatic air humidification
Figure 2. Adiabatic air humidification
The wet bulb temperature is measured by an ordinary thermometer wrapped in a moist cloth. Continuous evaporation of water causes the temperature to fall. The wet bulb temperature is always less than the dry bulb temperature, except when relative humidity is 100%, i.e. when the air contains the maximum quantity of water vapour possible at that specific temperature.
Below is an example of a direct evaporative cooling application in summer. In an air handling unit, the supply air, a mix of fresh air and recirculated air, is adiabatically humidified and is transformed from a temperature of 30 °C at 30%rh to 25 °C at 50%rh.
 Figure 3. Direct evaporative cooling
Figure 3. Direct evaporative cooling
Key:
A: supply air before humidification;
B: supply air after humidification;
The heat removed from the supply air can be calculated using the formula:
q
sens ≈ G
a · c
pa · (t
2 – t
1)
where:
q
sens = sensible heat, in kW;
G
a = air flow-rate, in kg/s;
c
pa = specific heat of dry air at constant pressure = 1.005 kJ/(kg · K).
In the example shown in the figure:
G
a = 30000 m3 /h · 1.225kg/m3 = 36750 kg/h -> 10.2kg/s;
t
A = 30 °C; tB=25 °C;
q
sens = 10.2 · 1.005 · (30-25) ≈ 51.255 kW.
The result of evaporative cooling depends on the starting temperature-humidity conditions. The arrows in the figure indicate different dry bulb temperature and relative humidity conditions.

Figure 5. wet pad applied to the coil
Figure 6. shade covers wetted by nozzles
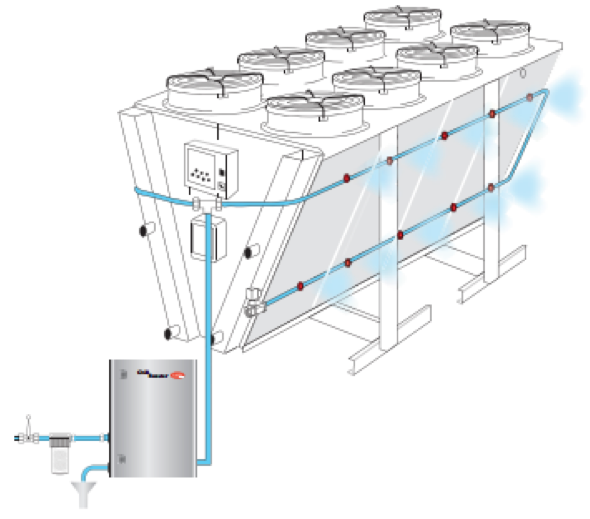 Figure 7. pumping unit and distribution system
Figure 7. pumping unit and distribution system
The water droplets evaporate spontaneously, absorbing energy from the air, which is consequently cooled, and comes into contact with the finned coil at a lower temperature. In this way, the heat exchanger can dissipate the rated amount of heat even when the climate is hotter than expected. Note that the atomized water particles do not evaporate completely before reaching the finned coil, both due to the short distance available and variations in outside air conditions; consequently, the heat exchanger fins are wetted, and this further increases overall system efficiency. Atomizers used for evaporative cooling can operate on untreated mains water or demineralized water. The advantages of mains water are ready availability and lower cost compared to demineralized water; against this, some of the mineral salts contained in mains water will accumulate on the fins, which therefore need to be cleaned periodically. Many manufacturers recommend against atomizing mains water more than 200 h/year so as to limit such fouling, a period that nonetheless is in line with peaks in demand. Demineralized water has a much lower mineral content, and therefore causes less fouling; manufacturers tend to agree that the units can atomize for up to 900 h/year before having to clean off mineral salts. The table below lists the advantages and disadvantages of the units illustrated in previous figures.
|
PROs
|
a)
|
b)
|
c)
|
|
Increased cooling capacity and smaller footprint, less usage of polluting refrigerants (gases), lower noise, reduced running costs
|
V
|
V
|
V
|
|
Coils not exposed directly to the sun
|
V
|
V
|
|
|
High heat exchange efficiency of coils that are kept clean (e.g. from dust, snow, sand, paper)
|
V
|
V
|
|
|
Lower condensing pressure and compressor discharge temperature, and consequently longer working life
|
V
|
V
|
V
|
|
Less compressor maintenance and greater system reliability
|
V
|
V
|
V
|
|
Cooling capacity at least 20-30% higher
|
V
|
V
|
V
|
|
Easy to install: simple assembly on existing (retrofit) and new units, without affecting the warranty
|
|
V
|
V
|
|
No water treatment required
|
V
|
V
|
V
|
|
Reduced environmental impact: lower power and water consumption
|
|
V
|
V
|
|
Supply air temperature around 5°C lower
|
V
|
V
|
V
|
|
CONs
|
|
|
|
|
High maintenance costs (change pad, covers)
|
X
|
X
|
|
|
Less precise control
|
X
|
X
|
|
|
Pressure drop all year round
|
X
|
X
|
|
Advantages of atomizing systems
Units equipped with atomizing evaporative coolers have many benefits when compared to traditional liquid coolers and condensers:
- no oversizing, smaller dimensions, lower air flow-rates, reduced fan and compressor energy consumption and less noise generated by the system;
- easy to install: the system can be easily installed on both new and existing air-conditioning and refrigeration installations. As the evaporative cooler is installed outside the building, no changes are need to the circuits inside the installation and the validity of the warranty on new or existing units is in no way affected;
- reduced environmental impact: lower power consumption (and corresponding CO2 emissions) due to the heat exchanger and less water consumption compared to evaporative cooling towers and wet pad coolers; in the latter case, for each liter of evaporated water around 3-4 liters are recirculated (hygiene problems) or wasted if using once-through units;
- if the atomized water is treated and purified there is less possibility of fouling or biofilm forming;
- the water inside the pipes may be heated by sunlight when the unit is not being used. The pumping unit manifolds face south and have a slight downward slope, and this, together with the automatic draining function that is activated whenever the pumping unit stops, helps prevent water stagnation. If there are any uncertainties surrounding possible bacterial contamination in the supply water, a special UV lamp kit can be used to improve the properties of the water;
- longer compressor life when used with condensers: lower compressor discharge pressure and condensing temperature diminish mechanical stress on the entire system, making operation more reliable and reducing maintenance.
Application
Below are manufacturer’s data relating to a dry cooler and the corresponding improvement in performance available through evaporative cooling, assuming fan speed is constant. Note that the heat dissipated decreases as the outdoor temperature increases, and that with evaporative cooling dry cooler capacity at 30 °C (365.9 kW) is comparable with the capacity at 25°C (385.5 kW) when dry, using 300 l/h of water. The equivalent temperature refers to the outside air temperature that can dissipate the same quantity of heat when dry. Assuming inverter-controlled variable speed fans are available, low temperature systems can achieve even higher efficiency.
| Design Data |
|
cooling water supply temperature (°C)
|
36
|
Coil area (m2)
|
1608.6
|
|
cooling water outlet temperature (°C)
|
31.3
|
Fin thickness (mm)
|
1
|
|
outdoor temperature (°C)
|
26
|
Fin material
|
Aluminium
|
|
outdoor humidity (%rh)
|
50
|
Air low rate (m3/h)
|
186600
|
|
power consumption (kW)
|
21.3
|
Heat dissipated (capacity)@ 26°C (kW)
|
340.3
|
|
Without evaporative cooling
|
With evaporative cooling
|
|
Outdoor temp
(°C @ 50%rh)
|
Dry capacity
(kW)
|
Wet capacity (kW)
|
Capacity increase (%)
|
Water
(l/h)
|
Equivalent temp (°C)
|
|
15
|
823.6
|
860.9
|
5
|
50
|
14.2
|
|
20
|
597.3
|
649.9
|
9
|
50
|
18.8
|
|
25
|
385.5
|
420.6
|
9
|
50
|
24.2
|
|
30
|
161.2
|
365.9
|
127
|
300
|
25.4
|
|
35
|
-
|
339.3
|
-
|
600
|
25.9
|
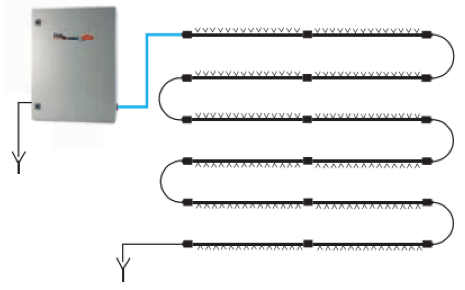 Figure 8. Layout of manifolds with nozzles to prevent water stagnation
Figure 8. Layout of manifolds with nozzles to prevent water stagnation
Note:
assuming fan and pump speed is constant, power consumption in all conditions is 21.3 kW;
all the capacity data in conditions other than design data (26 °C@ 50%rh) have been estimated using a special tool developed by CAREL.
Source: coolingbestpractices